Photocatalysis is a process that leads to the oxidation of various compounds under the influence of ultraviolet radiation and a properly selected catalyst that allows the entire reaction to take place. As a result of photocatalysis, various complex chemicals can be broken down into water, carbon dioxide and inorganic anions.
Therefore, a suitable catalyst and a suitable amount of light are needed to carry out the entire reaction. The substance that works best in this role is titanium nano dioxide, or TiO2. The problem, however, is that it can only absorb UV radiation. Although UV radiation is also present in visible light, its share is relatively small and does not exceed 5%. This means that for the effective activation of the process, it is necessary to either provide additional illumination with a UV-emitting device, or to increase the ability to absorb light by using various admixtures. The latter solution is more economical, therefore the surface of the titanium dioxide is modified with various other elements. In the case of titanium coat bacto vir shield, this additive consists of single silver atoms. Our unique manometric composition looks like a bunch of grapes, where the fruits are silver atoms and the stem is a nano-skeleton of titanium dioxide. This unique design ensures that the coated surfaces are protected day and night, without the need to supplement the coating with additional components.
How is the photocatalysis process going?
Under the influence of visible light, the modified titanium dioxide absorbs photons, which knock out electrons from the valence shell farthest from the atomic nucleus. This makes it possible, in the titanium dioxide semiconductor, to jump them from the baseband to the conduction band separated by a small band gap. Electrons passing to the conduction band can combine with oxygen, leading to the formation of its active forms – superoxide anion O2–. After donating an electron in titanium dioxide, positively charged electron holes are formed. This leads to a combination with water in the air and the formation of hydroxyl radicals.
Due to the lack of one electron, hydroxyl radicals are extremely chemically active and easily react with other compounds. They are able to connect, among others with volatile organic compounds floating in the polluted air, e.g. (PAH) benzoaprene, biphenyl or naphthalene, i.e. polycyclic aromatic hydrocarbons found in car exhaust fumes or harmful nitrogen oxides. This leads to the breakdown of more complex substances into ever simpler compounds. This activity is extremely effective against viruses and bacteria , breaking them down into water and carbon dioxide – substances that are not harmful to humans.
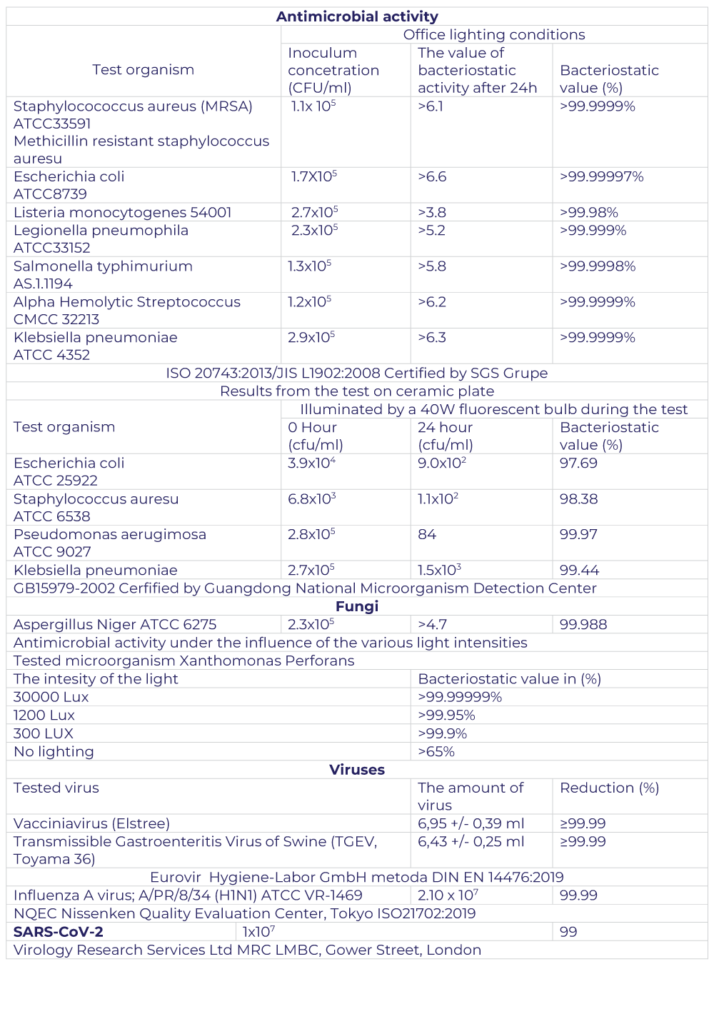
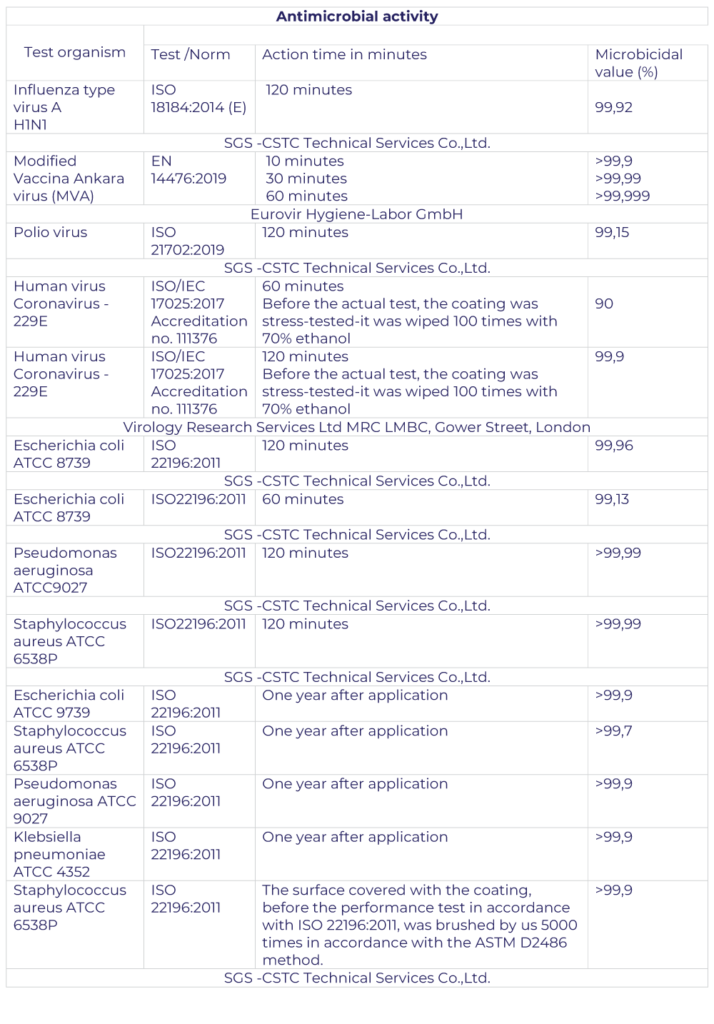
Confirmation of the effectiveness of TC:
Research of the Central Institute for Labor Protection: Study of the emission of nanoparticles during the processes of spray application of protective layers from an aqueous solution of nano titanium dioxide
- PZH approval Virucidal
- activity including SARS-CoV2 according to the EN 14476: 2019 method Virucidal activity on the material against influenza A H1N1 (A / PR / 8/34) virus from MDCK in accordance with ISO 18184: 2014 (E)
- Virucidal activity on a plastic surface against influenza A H1N1 virus (A / PR / 8/34) from the ATCC VR-1469 collection in accordance with ISO 21702: 2019
- Test for non-toxicity in accordance with FHSA regulation 16 CFR 1500.3
- Test for non-toxicity in accordance with US FHSH 16 CFR 1500.41
- Bactericidal activity against Legionella pneumophila according to the method of JIS L 1902: 2008
- Bactericidal activity against Listeria monocytogenes according to the method of JIS L 1902: 2008
- Bacteriological activity against methicillin resistant Staphylococcus aureus ATCC 33591 according to the method of JIS L 1902: 2008
- Test of the antibacterial properties of the coated nonwoven fabric against Salmonella typhimurium AA 1.1194 according to the ISO 20743: 2013 method
- Bactericidal activity against Alpha haemolytic Streptococcus (CMCC 32213) according to method ISO 20743: 2013
- NOx removal efficiency from air according to ISO method 22197-1: 2016
The tests were carried out in various lighting conditions and on various types of surfaces. Moreover, the coatings have also been tested for NOx reduction in air according to ISO 22197-1: 2016 and their effectiveness has been proven. TCs are also perfect as protective chemicals on solar panels or facades.